Research
Introduction
Mixing up friends and foes, or confusing prey with a predator, can be harmful to an individual or animal. To thrive in an ever-changing environment and make swift decisions, animals need to extract invariants, i.e., to form categories. Categorization, the process of grouping individual instances (individuals, thoughts, experiences) into broader classes sharing similarities, is a cognitive ability fundamental for survival throughout the animal kingdom.
Among distinct sensory modalities, audition is appealing yet overlooked to study categorization. Many species use their auditory system to parcellate complex acoustic scenes into useful perceptual objects guiding their future behavior (foraging, reproduction, threat avoidance). Yet, discovering the neuronal circuits and dynamics subtending auditory categorization is a challenge, as these circuits likely involve large parts of the brain and display complex brain-wide activity patterns.

At large, the lab's research project aims to establish how distributed neuronal circuits lead to categorization of natural sounds and ultimately guide behavior. We study this in mice, who rely heavily on their sense of hearing and are the species with the most advanced genetic toolbox that is available to study brain functions. We are designing innovative spatialized auditory stimulation systems to investigate the acoustic category repertoire of mice. We want to identify key brain-wide networks underlying sound categorization by recording thousands of neurons simultaneously across the brain and to analyze brain activity signals with machine learning algorithms. We also manipulate neuronal activity using opto- and chemogenetic tools. We also probe how emotional aspects of sounds influence auditory categorization using a naturalistic approach to behavior.
Together, this work not only shed novel insights into how the brain categorizes sounds to guide behavior but also further our understanding of how brain-wide mechanisms underlie cognitive processes. Below are developped the main scientific themes and techniques in motion in the lab.
Naturalistic Auditory Processing
Natural sounds vary across multiple auditory dimensions (frequency, amplitude), behavioral, and emotional dimensions. Animals need to be able to recognize the call of predators, prey, potential mates, natural sources of danger, and food sources to survive. Animals have most likely optimized the capacity to extract ethologically important acoustic invariants through evolution. The use of social sounds to understand auditory processing has led to important progress (song-selective neurons in songbirds and call-selective neurons in primates), but a systematic study of the response properties of neurons to natural sounds and the link to emotional processes is missing. The emotional dimension of natural sounds is often overlooked but will be investigated by this research agenda.
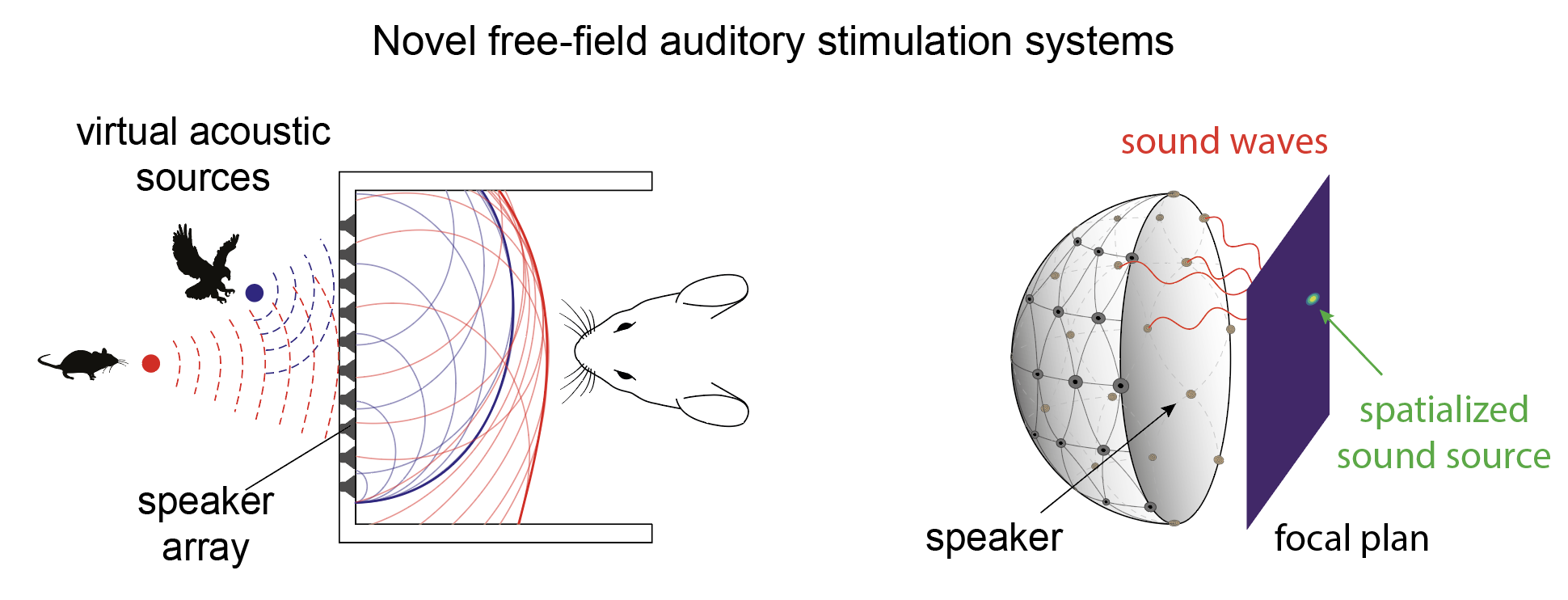
To bring complex natural panoramic auditory scenes into the laboratory, novel free-field auditory stimulation systems, beyond simple arrays of speakers and binaural systems, giving complete control over spectral, time, and spatial dimensions of the delivered stimuli, need to be designed. Advances in three-dimensional acoustic stimulation, usually developed for humans, can be used to generate spatialized three-dimensional sound sources for head-fixed and freely moving animals. Our rationale here is to design and develop spatialized auditory stimulation systems, beyond the state of the art, to investigate natural sound categories in mice.
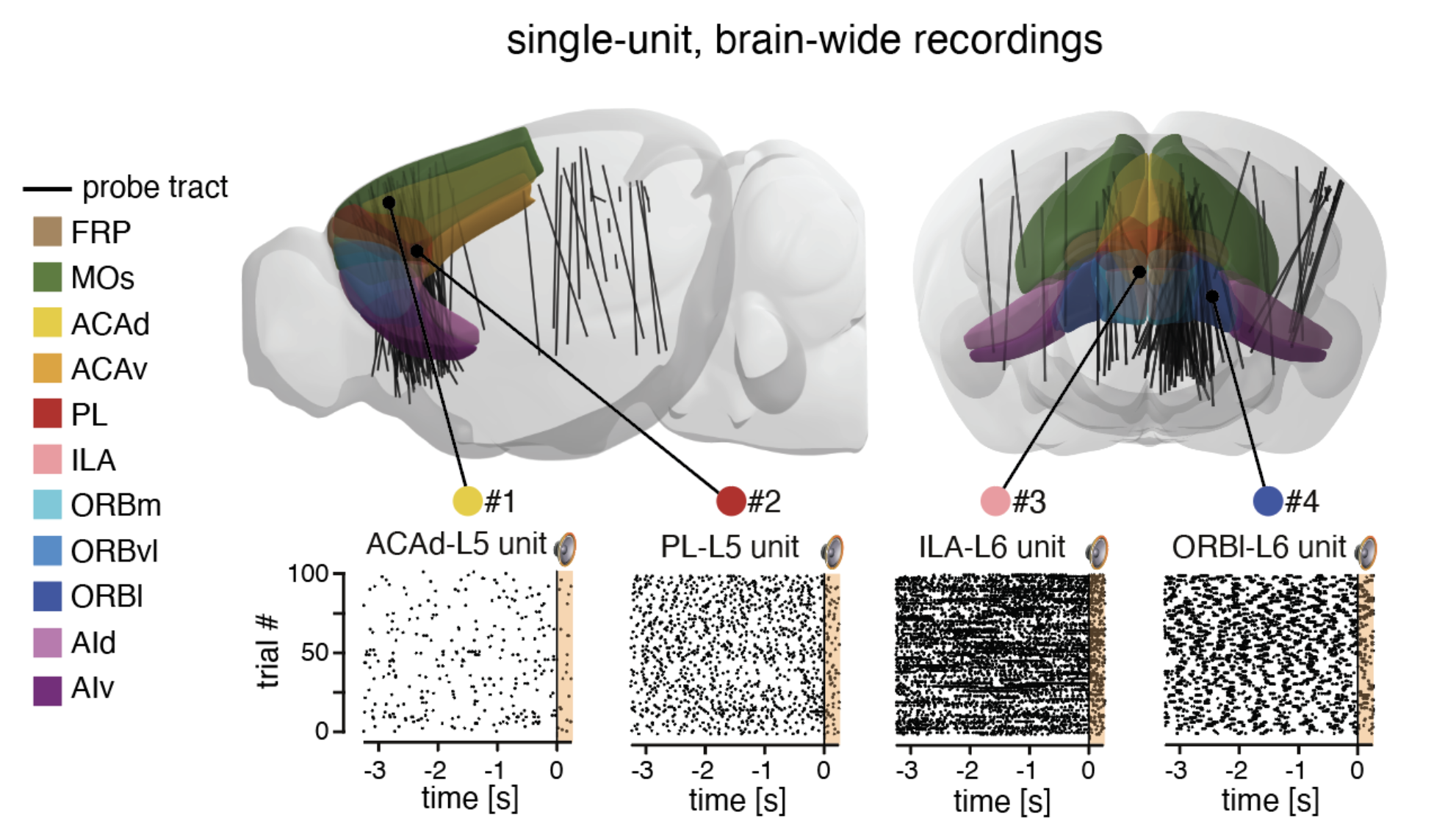
The anatomical pathways processing complex sounds are distributed across the brain. Currently, little is known about how distributed brain regions interact to form cohesive representations of sounds (and acoustic categories by extension). Previous reports suggest the involvement of a network of associative cortical brain regions, especially when natural complex stimuli are presented. We will perform simultaneous high-density electrophysiological recordings of selected brain regions relevant for auditory processing and auditory categorization.
Auditory Categorization
The act of categorization is one of the most basic human cognitive abilities and has been described by a variety of classic philosophers from Plato and Aristotle to Kant up to more recent thinkers. The classical view of categorization stems from cognitive linguistics where it is defined as the act of grouping single instantiations that share a degree of similarity (individuals,thoughts, experiences) into more abstract mental groups (categories). For instance, a specific animal (a single mouse) is taken as an instantiation of the category (here the species) Mus musculus. Because categorization is a powerful cognitive mechanism to organize knowledge of the world, it has been studied extensively by many research fields: psychology, ethology, linguistics, and ecology. In the last decades, neuroscientists joined the fray looking for neuronal underpinnings of categories in the brain.
The efficient coding hypothesis (Barlow) states that animals have optimized the capacity to extract ethologically important acoustic invariants through evolution. This theoretical framework motivates the search for behaviorally defined sound categories. Indeed, the ability to detect commonalities and differences from complex environmental scenes is observed across many animals: crickets, spiders, bees, crocodilians, birds, rodents, ferrets, and monkeys. As animals encounter various situations, the organisms and their nervous systems must dynamically adapt and decide ’what to do’. The overarching goal of this research program is to gain new insights into how categories allow to flexibly map the sensorimotor specific of a situation to actions using novel behavioral paradigm, more ecologically inspired.
Understanding how organisms extract complex auditory features from panoramic acoustic scenes to combine them into a coherent percept, is one central question in neuroscience (referred to, at large, as the binding problem). Answering this question based on animal models is even more challenging as they cannot verbalize how they perceive sounds. To circumvent this problem, we try to infer the processing of sounds from the neuronal activity. In the mouse, distinct sound categories have been decoded from auditory cortex neuronal activity. But does the sound category information decoded from neuronal activity in the auditory cortex come from local computations or does it originate from other connected cortical regions influencing the activity of the auditory cortex as suggested in general for distributed cognitive processes? Therefore the next steps for the identification of brain circuits underlying sound categorization require the investigation of distributed brain-wide networks.
Animal Cognition
The lab is interested to understand what cognition means in biological terms and how abstract cognitive variables are implemented by brain-wide networks. Brain circuits are continuously performing neuronal computations allowing organisms to act upon hidden rules of the external world. The capacity to extrapolate outcomes from limited observations, decipher rules from complex situations, and maintain focus on specific external inputs exemplifies the abstract cognitive operations performed by various species. By studying the neuronal activity of model organisms during well controlled behavioral paradigms, we hope to reveal the neuronal correlates underlying the abstract cognitive varibles. To achieve this goal we need to have a good knowledge of "the basics" i.e. how neuronal networks are anatomically arranged and how do they respond to simple sensory and motor information. With our current tools, we can approach the thorny problem of cognition in modern neuroscience.
As a new window into animal cognition, ccological-inspired neuroscience theories describe continuous sensorimotor interaction between an organism and a complex, ever-changing environment, which continuously offers opportunities and demands for action (referred to as ‘affordances’, Gibson). Through evolution, the organisms and their nervous systems have optimized how to select the appropriate action in a bewildering variety of situations. In the ecological-inspired neuroscience framework, categories are defined by mapping ‘what to do’: i.e., the action to execute, to the sensorimotor specifics of a situation: the sensory percept is the action to execute (e.g., I” know this object is a cup because I can drink with it”). The existence of ‘action-defined’ ethological categories can be tested under current controlled experimental conditions and could bring new insight into understanding mental abstractions, albeit basic neuronal computations underlying action-defined categories remain to be investigated. The overarching goal of this scientific proposal is to gain new insights into the neuronal underpinnings of categorization using a novel behavioral paradigm, more ecologically-inspired.
The prefrontal cortex
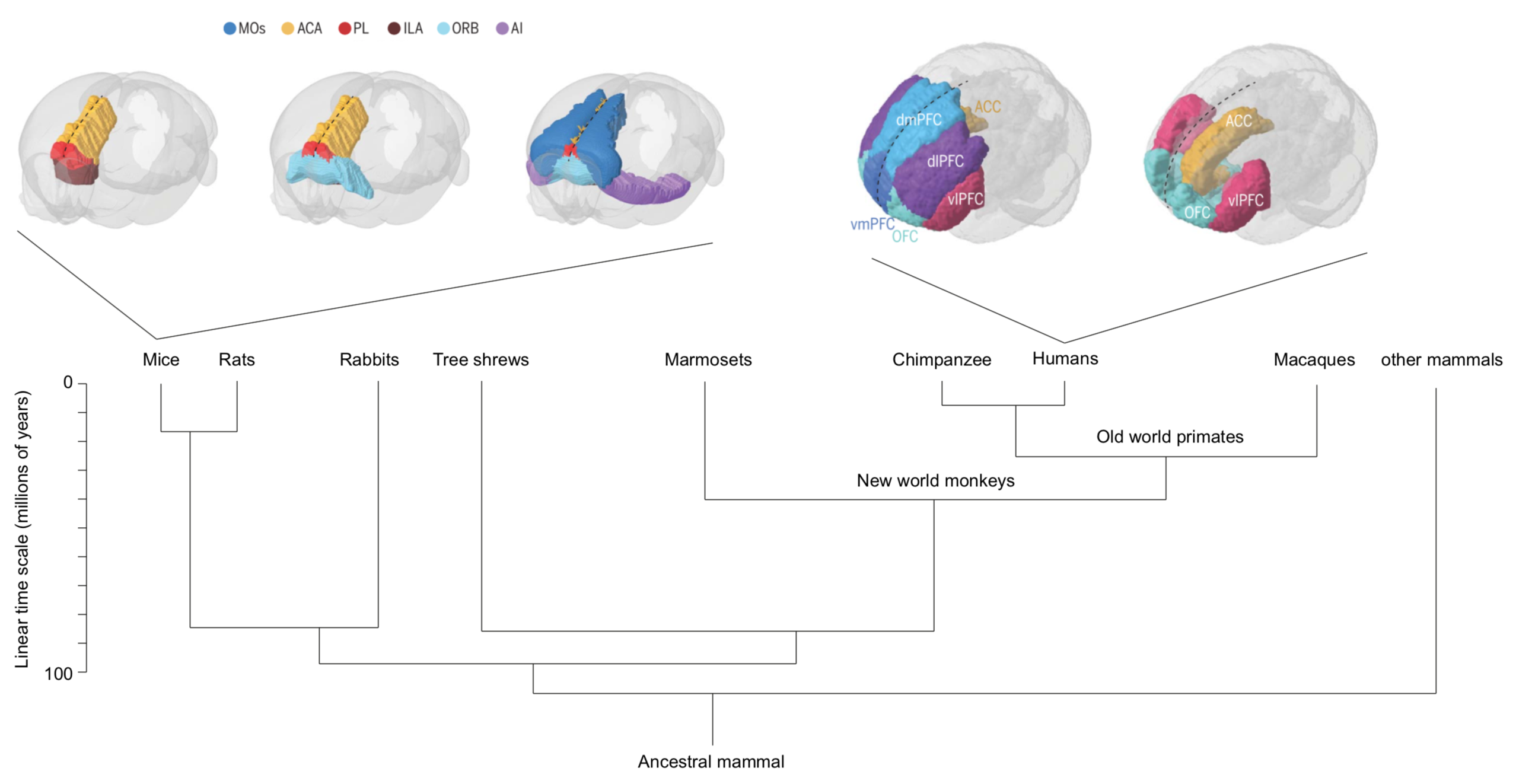
Across species, the prefrontal cortex (PFC) is identified as a key region for categorization. Previous work in humans, monkeys, and rodents establishes neuronal activity patterns related to categories, e.g., single neurons in the PFC showing category-specific activation. Nevertheless, no clear consensus is reached when comparing anatomical and functional homologies of the frontal regions across species.
PFC is an umbrella term referring to cortical regions located in the front of the brain. The PFC still lacks a conclusive definition, and the structure and function of this brain area across species remain unresolved. The PFC is implicated in perceptual, emotional, social, motivational, and numerous other brain processes and considered to enable flexible behavior. In following, disturbed PFC functioning has been connected to most, if not all, mental disorders, including drug addiction. Present-day preclinical researchers increasingly utilize mice (Mus musculus) as model animals. However, in parallel clinical transfer of pre-clinically identified therapeutics targeting mental disorder (and other brain disorders) is still at large failing. Lack of understanding of the structure and function of the brain hampers the understanding of which findings are transferable between species. Needless to say, deciphering of the structure and function of the PFC is of great importance to medicine. While class-common functions and class-common behaviors have been firmly established, effort must also be put into clarifying dissociations, and perhaps most so between primates and rodents. Ultimately, understanding of what makes the human PFC unique will build on comparative studies in different species.
Cytoarchitecture of the mouse (pre)frontal cortex
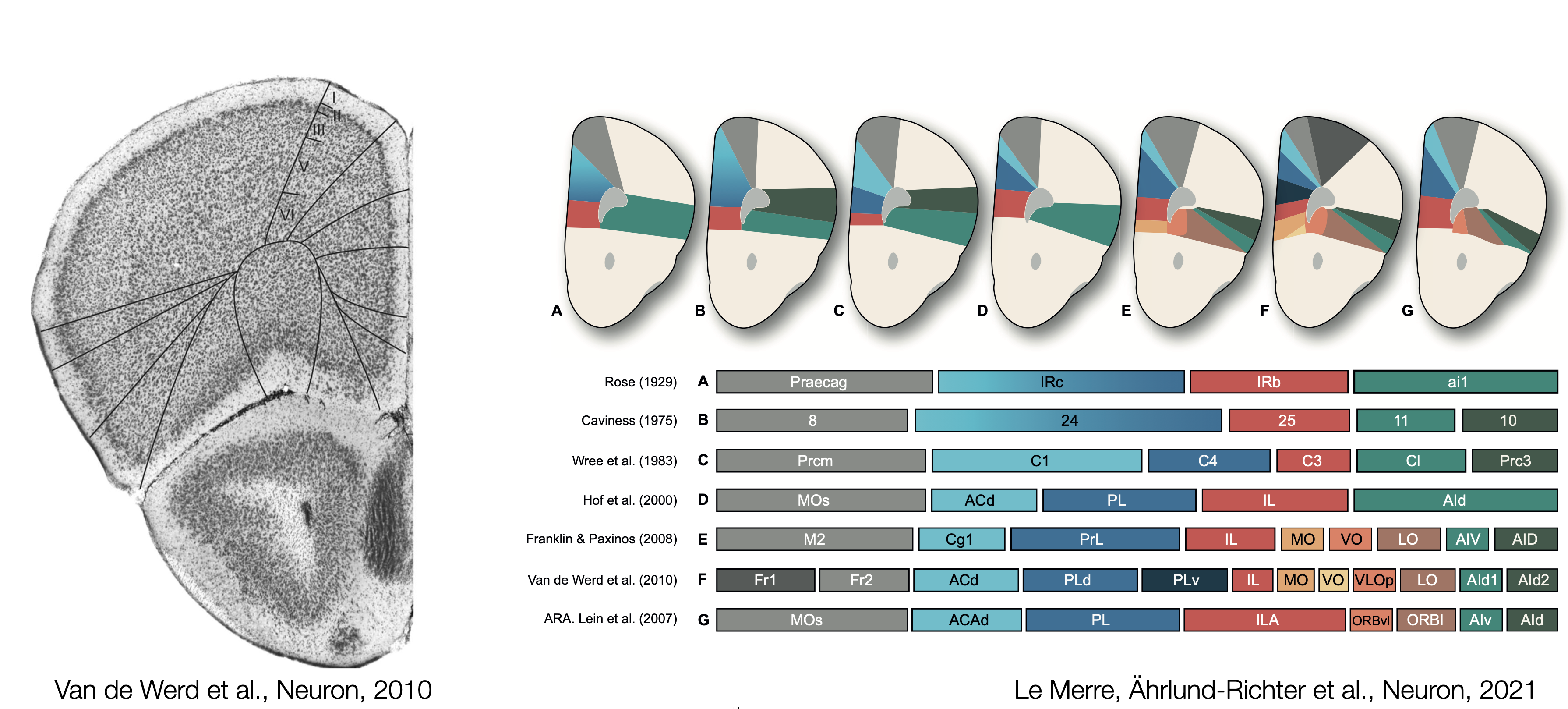
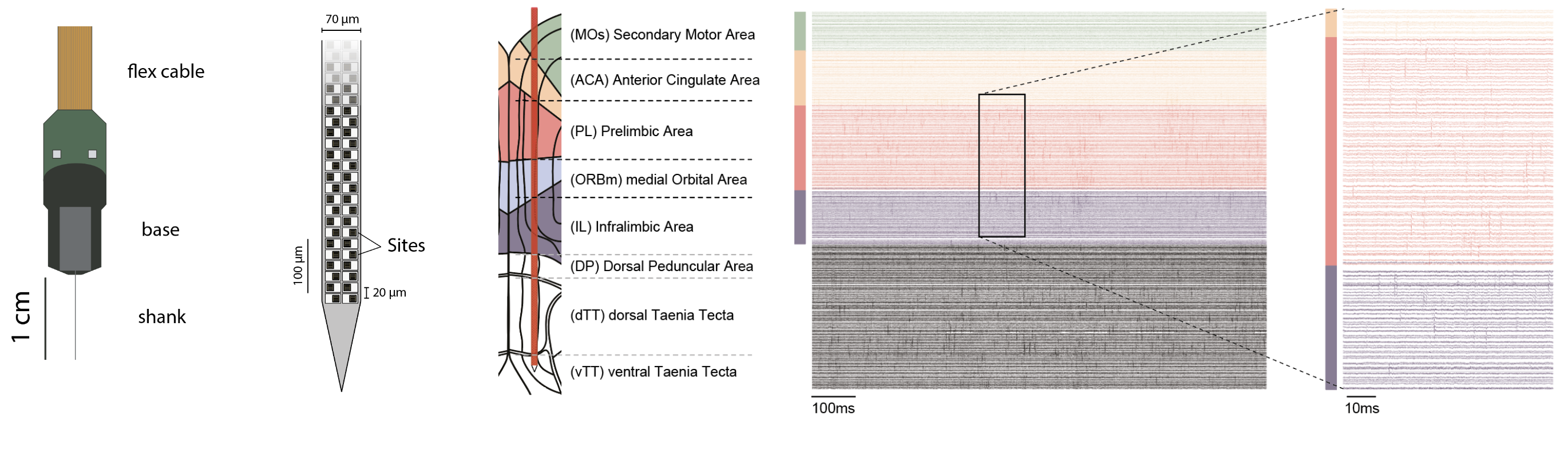
The first maps of the mouse prefrontal cortex come from cytoarchitecture and the seminal work of Korbinian Brodmann where the shape and the size of neurons determine brain regions. Recent atlases divide frontal regions into the Anterior Cingulate areas (ACA; dorsal and ventral), the Prelimbic area (PL), the Infralimbic area (ILA) and Orbital areas (ORB; medial, ventrolateral and lateral). These regions are flanked by the Secondary Motor area (MOs), the Frontal Pole (FRP), Agranular Insula areas (AI; anterior and ventral), the Dorsal Peduncular area (DP) and the Taenia Tecta (TT; dorsal and ventral). The caveat of cytoarchitectural patterns defining regions is the lack of reproducibility and agreement regarding the number and the borders of delineated regions. Over the last century, the names and borders of mouse prefrontal regions have kept changing.
Connectivity of the mouse frontal module
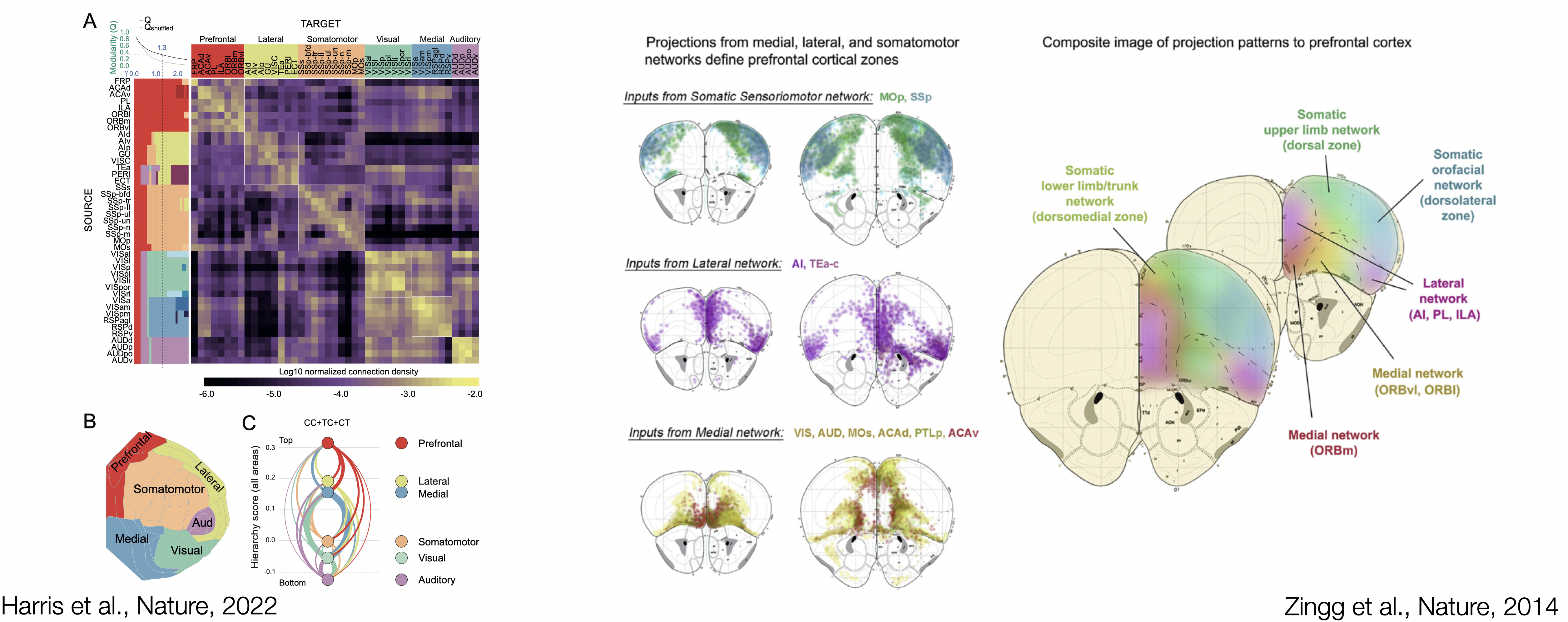
As neuron extend their axons across brain regions, they define the main roads that can be taken to exchange information. To establish how the prefrontal module communicate with the rest of the brain, the main neuronal pathways need to be identified. Viral delivery of fluorescent proteins into genetically targetted neuronal populations has become, over the last two decades, the standard technique to trace afferent and efferent connections of a brain region. The high diversity of mouse lines and the access to a large viral toolbox are the essential components for the prefrontal circuit dissection.
Recent work from the Allen Institute of Science has identified cortical modules based on connectivity patterns. Modules are brain region more interconnected with themselves than with the rest of the brain. The PFC stands as a coherent cortical module encompassing the Anterior Cingulate areas (ACA; dorsal and ventral), the Prelimbic area (PL), the Infralimbic area (ILA), Orbital areas (ORB; medial, ventrolateral and lateral) and the Frontal Pole (FRP). Interestingly, the Secondary Motor area (MOs), and Agranular Insula areas (AI; anterior and ventral) are the last one to jump out of the prefrontal module, potentially indicating that some parts of MOs and AI could be considered more "prefrontal". Taken together, the identified prefrontal module is the cortical module with the most extensive afferent and efferent connectivity with the rest of the brain, forming large brain-wide networks of connected regions.
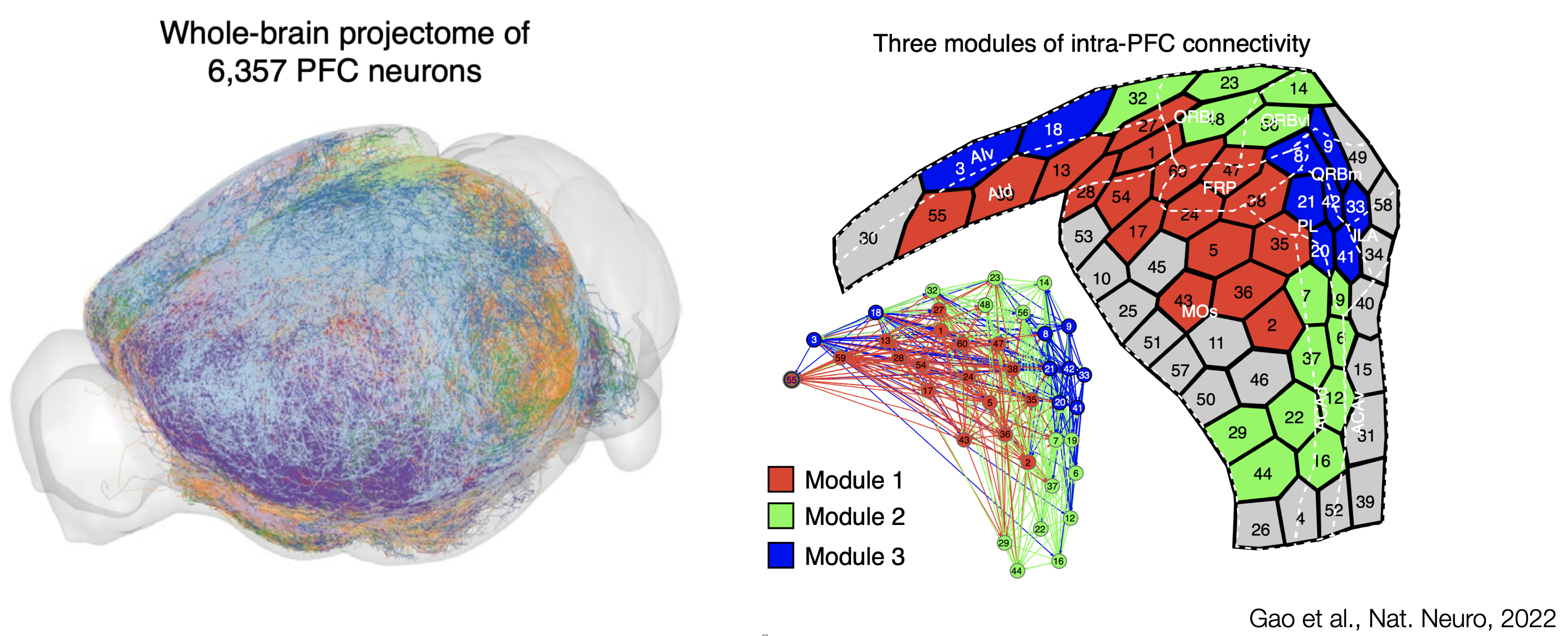
A single-neuron approach, tracing 6,357 single prefrontal neurons axonal projection, has recently been used to delineate interconnected modules within the mouse PFC. From the work of Gao et al., the main three connectivity-defined modules do not abide by cytoarchitectural boundaries. Connectivity eventually orchestrate brain activity by allowing information to flow between brain regions. The lack of agreement between cytoarchitecture and hodology questions the definition of prefrontal regions: What should we take into account to reach a comprehensive definition of the PFC?
Molecular maps of the mouse frontal cortex
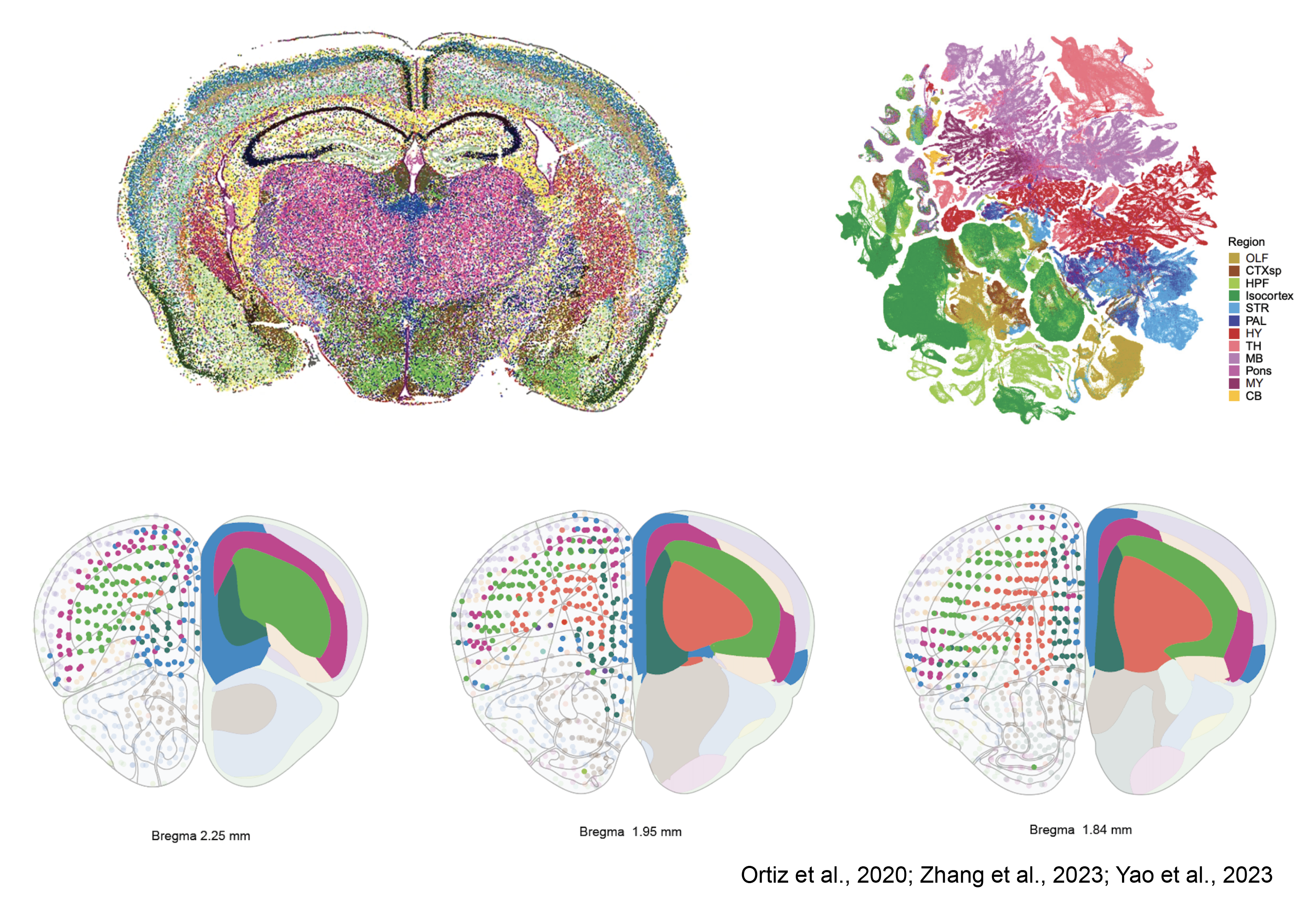
Mammalian brain regions embody three-dimensional architectures of neuronal assemblies established during development and evolution. Advancements in sequencing tools now enable us to discern the spatial patterns of gene expression profiles. In essence, brain regions and subregions are outlined solely based on their gene expression classification. This method unveils a laminar organization within the frontal cortex, aligning with what is traditionally recognized as the PFC. Thus, once again, the regions of the PFC identified by cytoarchitecture do not conform to the available gene expression maps. Despite being in the early stages of development, spatial transcriptomics and comprehensive genetic profiling of entire brain regions present a perplexing scenario. The lack of agreement between cytoarchitecture, connectivity and genetic identity remains to say the least, a puzzle that beckons further exploration.
Functional map of the prefrontal cortex
Can functional properties of prefrontal neurons help us to understand the cellular logic of prefrontal operations? Can we use functional properties of neurons to make a map of brain regions? My previous work in Pr. Carlén's laboratory has been focused on new methodological and analytical approaches to establish functional maps of the mouse prefrontal cortex. Here are some of the results we obtained.
Research synthesis of mouse prefrontal functions
The prefrontal cortex has been ascribed many functions. in a recent perspective article, we have summarized the last decade of mouse PFC research. The data and methodology used to perform the functional research synthesis presented in the figure 3 of our perspective article: The mouse prefrontal cortex: Unity in diversity. The code used to generate the figures can be found on this github repository
Database
The database used to perform our research synthesis is available as a table. This table has the following columns: the year of the study, the short publication reference, the PFC region name used in the original publication, the inactivation method used, the stereotaxic triplet provided in the original article. The averaged coordinates used for the 3D plot into the Allen reference Atlas (ARA) CCFv3, the correction applied to the DV value to translate it into the ARA (see also below), the 3 scores; complexity index, sensory modality and task type, obtained for every publication, 7 logical values (in MOs, in ACA, in PL, in ILA, in ORBm, in ORBvl and in ORBl) indicating in which PFC region the study is found when plotted into the ARA CCFv3 with our method and the name of the Allen PFC region containing the stereotaxic triplet provided in the original publication.
The full database table can be downloaded here as an Excel or csv file.
3D visualisation
Here is an attempt of 3D visualisation of the database using plotly. Some improvements are still required. The Task type and Sensory Modality parameters are displayed as continuous variables although they are discrete variables. You can mouse over the dots in the scatter plot to see from which study is the data ONLY if you have already scrolled within the brain mesh (plotly feature). We hope to improve this 3D visualisation with time.
Limitations
The CCFv3 version of the Allen Atlas is a 10um voxel resolution space and our analysis using this Atlas has some limitations. We generated 3D meshes for the brain outline, for each PFC region (MOs, ACA, PL, ILA, ORBm, ORBvl and ORBl) and for the PFC sudivisions described in the section of our Perspective “Parcellations of the PFC” (dmPFC, vmPFC and vlPFC) by using the MATLAB “isosurface.m” function (see folder Brain_meshes). To detect in which Allen PFC region each publication was peformed we used the “inpolyhedron.m” function (Copyright © 2015, Sven Holcombe). The thickness of the borders in the actual version of the CCFv3 is around 30um (3 voxels). As a result some triplet of coordinates are allocated to 2 regions while performing our “inpolyhedron.m” detection procedure. We inspected manually each triplet of coordinates whithin its respective ARA plate (see folder AllenAtlasLocationCCFv3_individualstudies) and visually assesed for these ambiguous cases to which Allen PFC region they belong to. Our level of incertitude regarding PFC region assignment needs to be put into perspective because, as accurate as we can be as experimenters, the provided stereotaxic coordinates in every publication are only averaged values reflecting the mean location of the targetted brain structures. This experimental incertitude is by itself an important limitation of our analysis and needs to be taken into consideration.
Source
2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas (2015) with region annotations (2017).
Functional maps fo the mouse prefrontal cortex
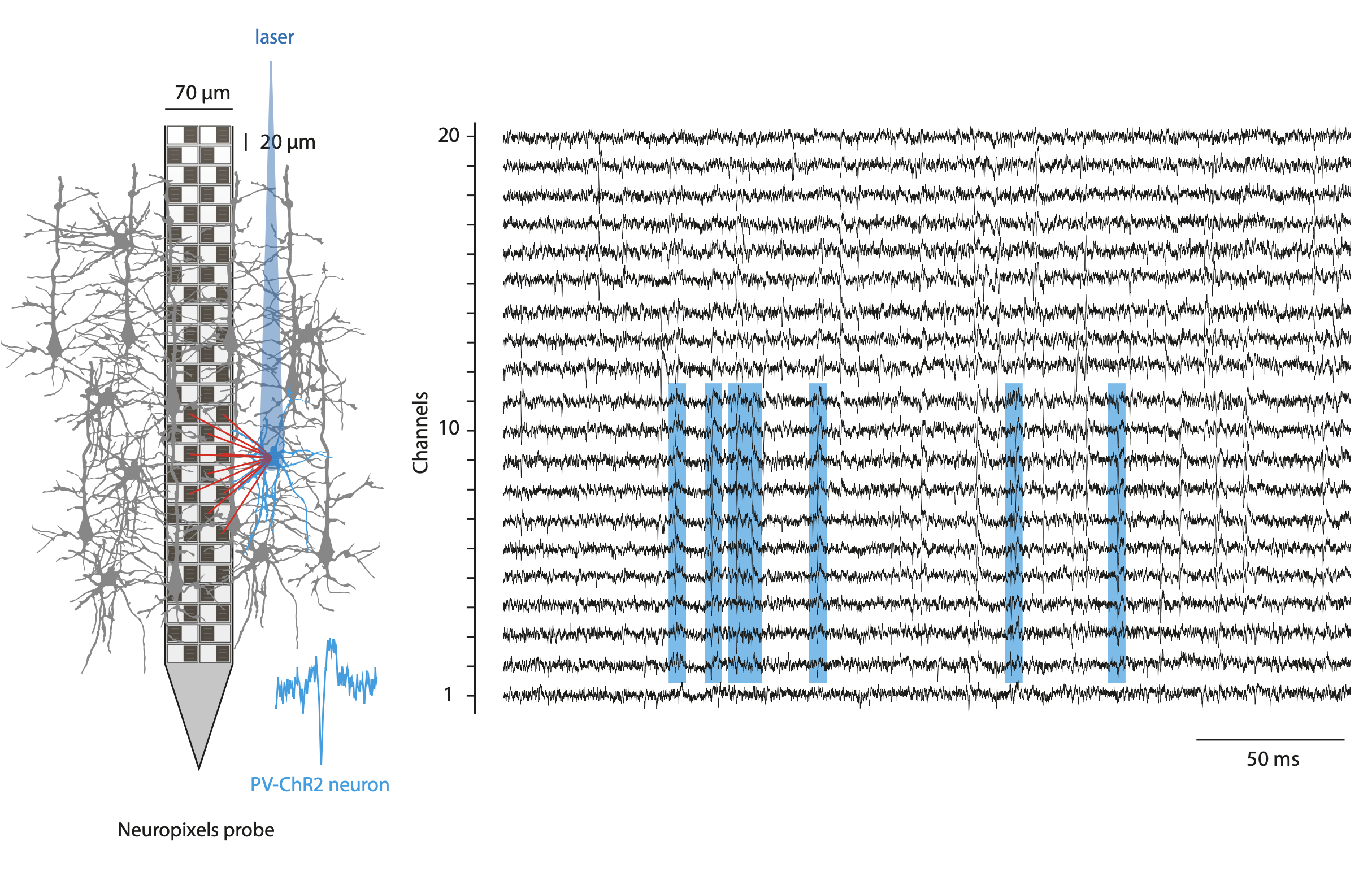
In this work, we used single neuron activity features (action potential dynamics) to parcellate PFC regions using the 3D annotated volume of the Allen Reference Atlas (CCFv3). To record simultaneously the activity of hundreds of neurons from different prefrontal regions, We used Neuropixels probes. These fully-integrated silicon CMOS digital neural probes (IMEC) allow to sample the extracellular neuronal activity simulataneously on 384 channels at a millisecond resolution. Combined with offline automated spike sorting algorithms, they offer the opportunity of recording units form many prefrontal regions in vivo. We use this system to gain knowlegde about the functional properties on the mouse prefrontal cortex regions while the mice engage in various behavioral tasks.
We identified functional neuronal types solely described by their spontaneous firing properties using self-organizing maps. For a given brain region, neurons are enriched within distinct territories of the activity landscape, describing functional fingerprints for brain regions. Prefrontal regions are characterized by a regular discharge pattern with a high memory index value. An observation consistent across other large-scale datasets (International Brain Laboratory). These results indicate the existence of a functional signature for prefrontal neurons. These functional fingerprints can be used to redraw the borders of the PFC. Interestingly, the newly obtained maps disagree with classical cytoarchitecture-based parcellations of the PFC and dynamically change upon sensory stimulation (auditory stimulus).
We want now to understand how the identified functional neuronal types are getting recruited during auditory categorization and how the functional maps of the PFC dynamically change as we engage animals in cognitive tasks.
Optogenetic manipulations
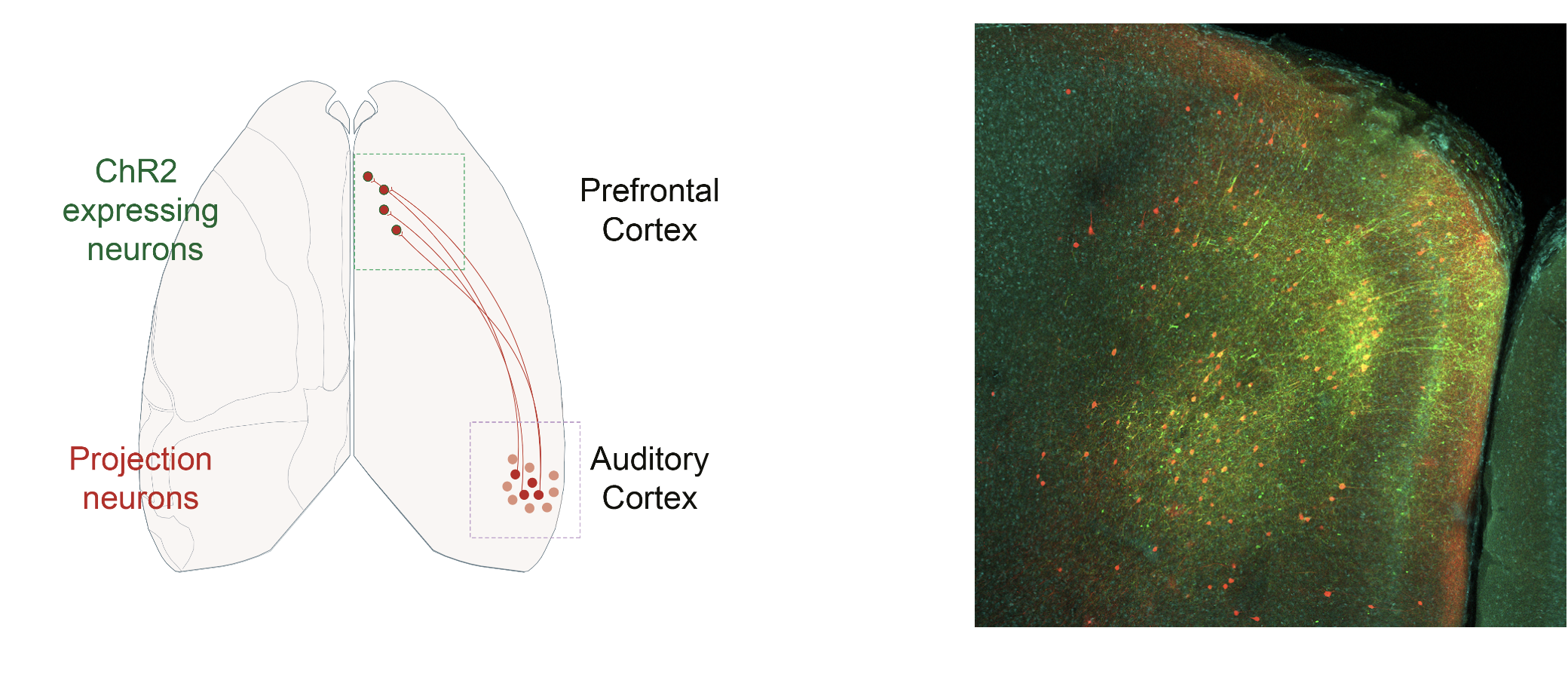
As one of the classical technique utilized in neuroscience, I use optogenetics manipulations to interrogate the role of the prefrontal regions and cell types of interest. I have principaly used the optogenetic approach to photoinhibit cortical regions and to optotag neuronal populations of interest.